Bristol-Myers stop kidney cancer drug tests after early success
(Reuters) – Bristol-Myers Squibb Co said on Thursday the success of its combination therapy to improve overall survival in kidney cancer patients helped it end the trial well ahead of schedule, despite earlier reporting mixed results on other main goals.
The company said a combination of Opdivo and Yervoy, its two main drugs, showed superior overall survival rates than a standard-of-care drug in previously untreated patients with advanced or metastatic renal cell carcinoma.
Last month, Bristol-Myers said the combination treatment had failed to improve progression-free survival in patients, but had succeeded in reducing the size of their tumors.
“We believe the overall survival benefit likely confirms the durability of the signal seen on progression-free survival, leading to a very high likelihood of approval,” Leerink Research analyst Seamus Fernandez wrote in a client note.
The trials to monitor overall survival rates were to run through the second half of 2019, according to Cowen and Co.
Bristol-Myers said the study was stopped early after the successful results in a planned interim analysis, sending its shares up 4.2 percent to a 52-week high of $62.415.
Exelixis Inc, which is developing a rival kidney cancer treatment, tumbled 10 percent. But, Bristol-Myers’ trial results raised hopes for AstraZeneca Plc’s lung cancer therapy that, in July, failed to show progression-free survival.
Barclays analysts said Bristol-Myers’ trial provides perhaps the most explicit evidence to date that progression-free survival may not be the best yardstick to measure the benefit of immunology-oncology drugs.
That is why, Bernstein analyst Timothy Anderson said, most trials now incorporate overall survival as a primary endpoint.
Still, Bristol-Myers’ Opdivo failed to prolong survival in previously untreated patients with non-small cell lung cancer, the largest cancer market, according to test results earlier this year.
The failure led to Merck & Co Inc’s Keytruda getting a leg up in the key immuno-oncology field and Bristol-Myers’ becoming the target of activist investors.
U.S. health regulators on Wednesday placed a partial hold on three trials testing Opdivo in combination with other medicines for multiple myeloma due to risks seen in similar studies on a rival drug.
Cowen and Co analysts estimate Opdivo could generate sales of $12.36 billion in 2022, of which renal cell carcinoma would account for about $1.7 billion.
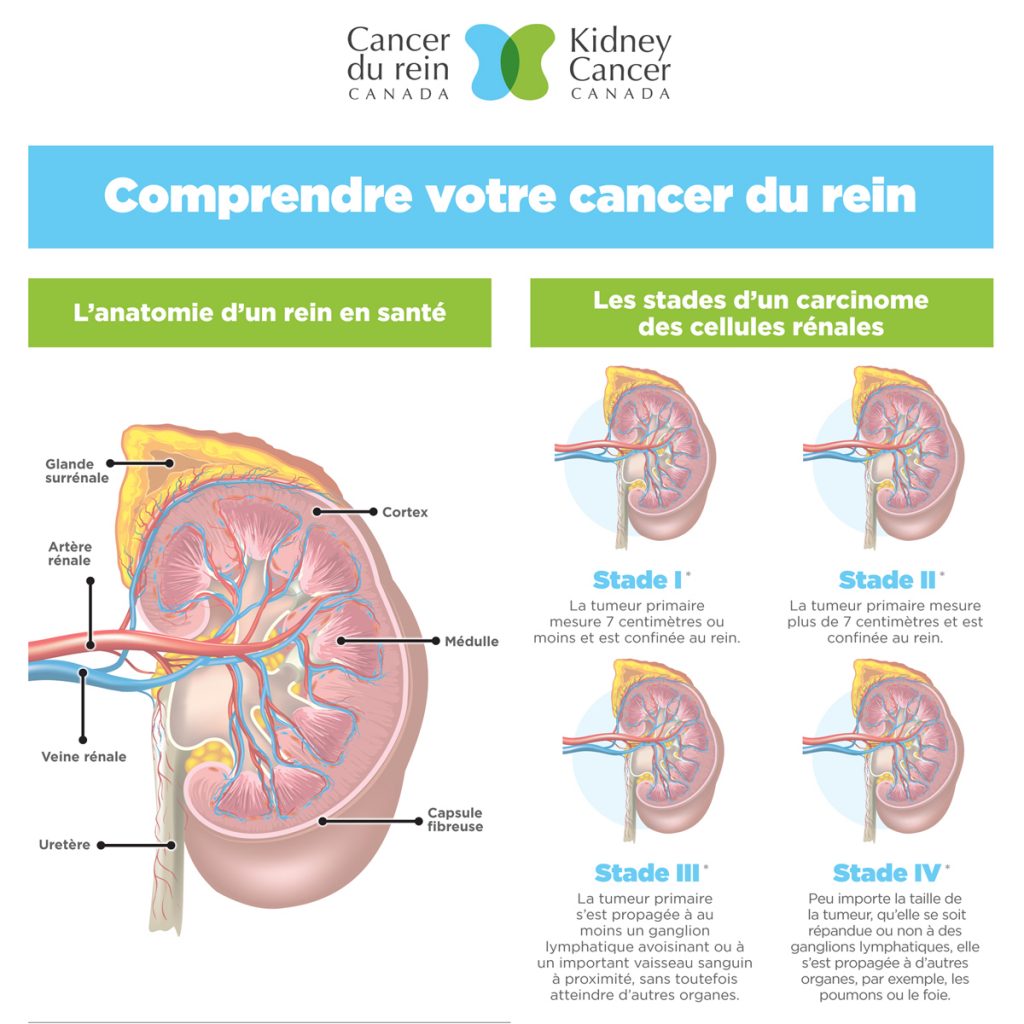
Renal cell carcinoma is the most common type of kidney cancer in adults, accounting for more than 100,000 deaths worldwide each year, Bristol-Myers estimates.
Reporting by Divya Grover in Bengaluru; Editing by Savio D’Souza
Our Standards:The Thomson Reuters Trust Principles.