The study of genetics and genomics in kidney cancer has begun to revolutionize how this disease is diagnosed and managed. Raymond Kim, MD, PhD, a Medical Geneticist and Medical Director of Cancer Early Detection and the Bhalwani Familial Cancer Clinic at Princess Margaret Cancer Centre in Toronto, provided an overview of how genetics and genomics impact kidney cancer diagnosis and management today, as well as a peek into what we might expect to see in the future.
Highlights from the webinar
Genetic mutations can be inherited or acquired over the lifetime
In the context of kidney cancer, genetics refers to the impact that specific genes have on this disease, while genomics refers to the impact of our entire genetic makeup. Cancer, explained Dr. Kim, is caused by genetic changes, or mutations, leading to uncontrolled growth of cells. These mutations can be hereditary, meaning you were born with them, having inherited them from your mother and/or father. These are known as germline mutations. You probably know that Angelina Jolie had her breasts removed to prevent the development of breast cancer. This is because she inherited a mutation of the BRCA1 gene from her mother, which is known to dramatically increase the risk of developing breast, ovarian, or other cancers. Genetic mutations leading to cancer can also be acquired. Lung cancer, for instance, is often triggered by mutations that develop from smoking. These acquired genetic changes are known as somatic mutations.
Dr. Kim specializes in germline, or inherited, mutations that increase the likelihood of developing certain kinds of kidney cancer. There are various ways of identifying the presence of such mutations. One way is to take a biopsy, or small sample, of a tumour and examine features of the cells under a microscope as well as conduct genetic tests to identify the presence of specific mutations. Another way is to conduct genetic testing on blood or skin cells, even if the cancer is not currently present in the blood or skin. This is because genetic mutations that are present at birth may be present in the skin or blood, even if those tissues are not currently affected by cancer.
An intriguing technology is a type of test known as liquid biopsy. Still largely in the investigative stage, it involves examining the white blood cells, known as the “buffy coat” in a blood sample, for evidence of cell-free DNA. These are pieces of DNA that have broken away from dead or dying cells and found their way into the bloodstream. Because cancer results in the rapid propagation of cells that quickly die off, only to be replaced by more and more cancerous cells, the presence of cancer anywhere in the body can result in high levels of cell-free DNA in the blood. Measuring these levels can therefore help diagnose cancer. When repeated over time, this test can also help determine whether a patient is responding well to therapy and whether an early recurrence is present, requiring a need to return to treatment following remission.
Identifying genetic mutations helps direct kidney cancer management
It is important to identify which genetic mutations are present in a patient with cancer because this can be used to direct treatment. Experts have developed specific, targeted therapies that directly address the changes in the normal cell cycle that are produced by cancer-causing mutations. This relatively new type of treatment is known as precision medicine, and there are several drugs of this type that are in use today or in clinical trials for use in the future. Until recently, precision medicine largely addressed somatic mutations, but they are increasingly being used for germline mutations as well.
Another important reason to identify mutations is that their presence provides unique insight into how likely an individual is to develop certain types of cancer. This helps with surveillance, or planning how often a person should undergo scans or other tests that detect the presence of cancer. In some cases, a germline mutation in a cancer patient may indicate the need to test other family members for the same mutation.
Dr. Kim and his team test eligible patients for 15 genetic mutations that have been definitively linked with kidney cancer. This number will increase over time as new mutations are identified. Every province has slightly different criteria for who should receive genetic testing for cancer genes. In general, however, the indicators that it might be necessary include a) diagnosis with a rare cancer; b) diagnosis with a type of cancer that usually affects people much older (e.g., more than 10 years); c) strong family history of certain cancers; d) multiple cancers in one person; and e) diagnosis with a specific subtype of cancer that is usually linked with genetic mutations.
Dr. Kim noted that it is important to differentiate the medical grade genetic testing that occurs within specialized medical centers and the genetic testing available online, which is not medical grade and more for “entertainment purposes” than guiding medical care. He recommended that people diagnosed with cancer who believe they or their families might benefit from genetic testing should discuss this with their oncologist. It is virtually always preferable to first test the person with cancer prior to determining whether genetic testing also needs to take place in family members, he said.
Genetic testing for kidney cancer is underused in Canada and the US, according to Dr. Kim. The process is currently being streamlined, with oncologists able to order genetic testing directly rather than having to first refer to genetic specialists. In order to provide some reassurance to those told they need genetic testing but who may be reluctant, Dr. Kim addressed key myths:
- Myth 1: Patients with genetic mutations associated with cancer will face discrimination.
Fact: In Canada, Bill S-201 makes it illegal for vendors of goods and services, such as life insurance, to discriminate based on genetics. In addition, prospective employers cannot ask you about your genetic status.
- Myth 2: Patients with genetic mutations associated with cancer will face discrimination.
Fact: In Canada, Bill S-201 makes it illegal for vendors of goods and services, such as life insurance, to discriminate based on genetics. In addition, prospective employers cannot ask you about your genetic status.
- Myth 3: Patients must pay for genetic testing.
Fact: If deemed medically necessary, most provinces will cover it. In addition, the cost associated with genetic testing is rapidly diminishing.
- Myth 4: It is taboo to have inherited forms of cancer.
Fact: This may have been true in the past, but with increased awareness led by celebrities like Angelina Jolie going public with her story, this taboo is largely becoming a relic of the past.
Kidney cancer syndromes associated with germline mutations are rare but devastating
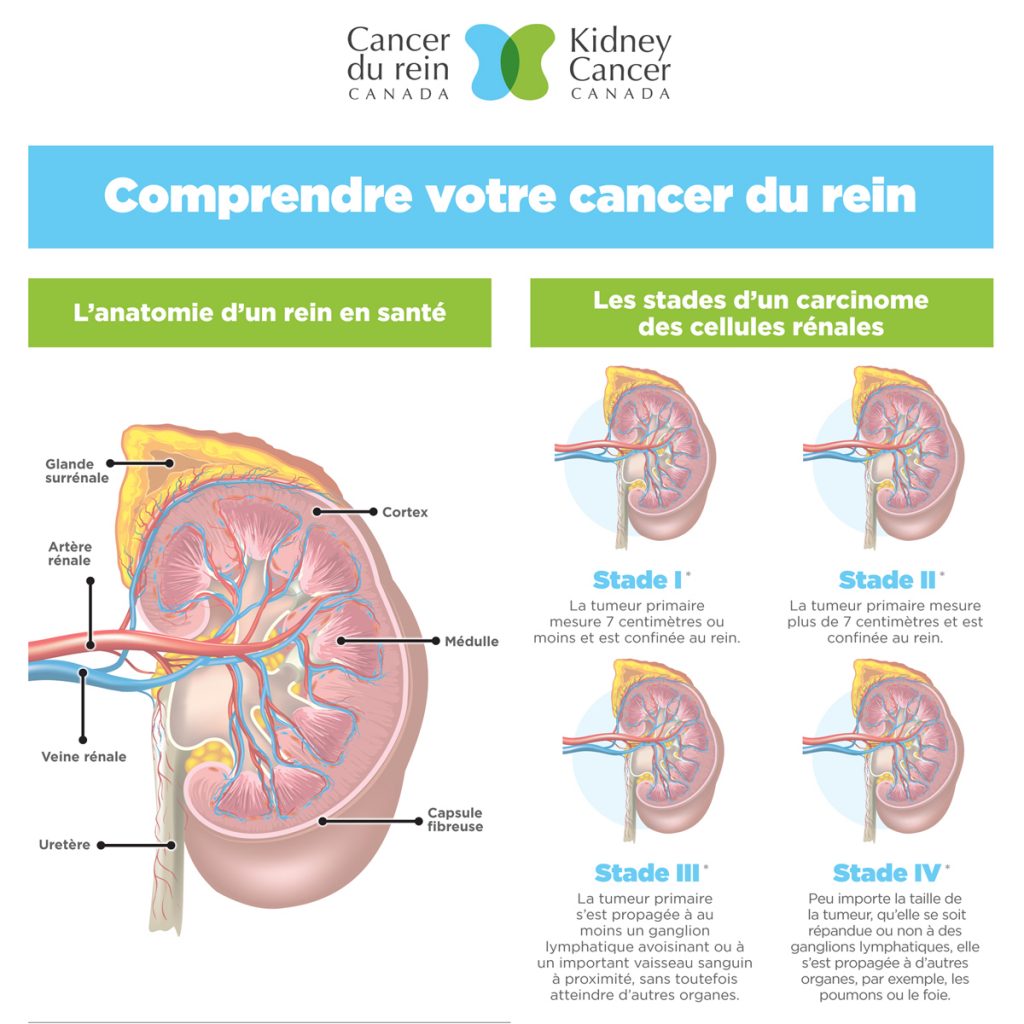
Dr. Kim wrapped up his talk by discussing some kidney cancer syndromes that are known to be related to germline genetic mutations. The term “syndrome” refers to a constellation of features that occur together. In each case, this includes the histological features of the cancer. Cancer histology refers to what the tumour cells look like under a microscope. Clear cell histology is the most common type of kidney cancer, while papillary and chromophobe histologies are far less common and also far more likely to be associated with germline mutations. The syndromes described by Dr. Kim were:
- Hereditary papillary renal cell carcinoma: This cancer occurs as a result of a mutation in the MET gene. It can be identified early via abdominal imaging, and these tumours mercifully grow slowly. The risk of developing this cancer diminishes with age.
- Hereditary leiomyomatosis and renal cell cancer (HLRCC): This is caused but a mutation in FH gene. In addition to producing papillary kidney cancer, it can cause fibroids in young women, which can be symptomatic enough to require treatment. If both parents have the FH mutation and both pass it on to their child, that child can develop a severe metabolic condition called fumarate hydratase deficiency.
- Birt-Hogg-Dubé syndrome: Caused by a mutation in the FLCN gene, people with this syndrome are at increased risk of developing types of kidney cancer known as hybrid oncocytic tumours (HOT) and chromophobe renal cell carcinoma (chRCC). Thy can also develop dome-shaped bumps on face called cutaneous fibrofolliculomas and may develop cysts in their lungs, which can rupture and cause pneumothorax, or a collapsed lung.
- Von Hippel-Lindau syndrome (VHL): A mutation in the VHL gene places patients at increased risk for clear cell carcinoma type kidney cancer and can also produce abnormal blood vessels in brain called hemangioblastomas as well as cysts in ears and pancreas, and tumours on the adrenal glands. A type of precision therapy known as belzutifan is used to treat this condition.
- Tuberous sclerosis complex (TSC): This multisystemic disorder is caused by mutations in the genes called TSC1 and TSC2. It can leas to lesions of the skin, cysts in the lungs, growth around nails, seizures, brain tumours, and noncancerous tumours in the kidneys that can unfortunately develop into cancer. This is syndrome is treated with a precision medicine therapy known as everolimus.
Fortunately, these syndromes are rare, occurring in anywhere from 1 in 20,000 to 1 in 200,000 people. This rarity makes them difficult to study, however. To address this, Dr. Kim and his team at the OHCRC are developing several strategies to bring medical information on all these patients into one centralized database, develop surveillance guidelines, and promote research on how best to manage these diseases. Since there is considerable overlap between these syndromes and more common forms of kidney cancer, this research will likely help improve the lives of people with all forms of kidney cancer.
 RAYMOND KIM, MD/PhD, FRCPC, FCCMG, FACMG
RAYMOND KIM, MD/PhD, FRCPC, FCCMG, FACMG
Raymond Kim received his MD/PhD from the University of Toronto with Dr. Tak W. Mak in Medical Biophysics. He then completed a residency in Internal Medicine, followed by a fellowship in Medical Genetics at The Hospital for Sick Children. His clinical interests lie in the transition of care, complex multidisciplinary care and adult hereditary disorders. His research incorporates novel genomic technologies in clinical care including whole genome sequencing and circulating DNA. He is Medical Director of Cancer Early Detection and the Bhalwani Familial Cancer Clinic at Princess Margaret Cancer Centre. He is also the PI of the Ontario Hereditary Cancer Research Network at the Ontario Institute for Cancer Research and Provincial Head of the Provincial Genetics Program at Ontario Health.


 RAYMOND KIM, MD/PhD, FRCPC, FCCMG, FACMG
RAYMOND KIM, MD/PhD, FRCPC, FCCMG, FACMG